Sexual Dimorphism in Estimated Stature from Long Bones in Gilimanuk, Semawang, Plawangan, and Recent Sample in Indonesia
Janatin Hastuti
 https://orcid.org/0000-0001-8621-463X
https://orcid.org/0000-0001-8621-463X
Lab. of Bio- & Paleoanthropology, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Medika St, Sekip, Yogyakarta 55281, Indonesia
Department of Nutrition and Health, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Farmako St, Sekip, Yogyakarta 55281, Indonesia
Ashwin Prayudi
 https://orcid.org/0000-0001-5461-8840
https://orcid.org/0000-0001-5461-8840
Lab. of Bio- & Paleoanthropology, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Medika St, Sekip, Yogyakarta 55281, Indonesia
Neni Trilusiana Rahmawati
 https://orcid.org/0000-0002-9686-0907
https://orcid.org/0000-0002-9686-0907
Lab. of Bio- & Paleoanthropology, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Medika St, Sekip, Yogyakarta 55281, Indonesia
Department of Nutrition and Health, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Farmako St, Sekip, Yogyakarta 55281, Indonesia
Noorman Hendry Fauzi
 https://orcid.org/0009-0005-0086-9800
https://orcid.org/0009-0005-0086-9800
Lab. of Bio- & Paleoanthropology, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Medika St, Sekip, Yogyakarta 55281, Indonesia
Rusyad Adi Suriyanto
 https://orcid.org/0000-0002-3820-0759
https://orcid.org/0000-0002-3820-0759
Lab. of Bio- & Paleoanthropology, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Medika St, Sekip, Yogyakarta 55281, Indonesia
Department of Medical Forensic, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Farmako St, Sekip, Yogyakarta 55281, Indonesia
Abstract. The study of sexual dimorphism among ancient skeletons can provide information on community health in the past. Meanwhile, the younger geological age of skeletal remains from Gilimanuk, Semawang, and Plawangan have received little attention. This study aimed to evaluate the sexual dimorphism in estimated stature of Gilimanuk, Semawang, Plawangan, in addition to a recent sample, of long bones. Observations were conducted on 44 (16 males, 28 females) skeletal remains of Gilimanuk, nine of Semawang (five males, four females) and 11 of Plawangan, (four males, seven females), and nine of recent (four males, five females) human skeletons stored at Universitas Gadjah Mada, Yogyakarta, Indonesia. Stature was estimated from the length of long bones. The highest average stature in the ancient sample was for Gilimanuk females (168.74 ± 9.18 cm) and males (174.10 ± 9.42 cm) in the age 16–<20 years. However, the averages of estimated stature in all ages were similar in both sexes. The average estimated stature of Semawang and Plawangan remains was slightly lower than those of Gilimanuk remains, i.e., 162.60 ± 3.97 and 159.08 ± 1.59 cm, respectively. In comparison, the recent human skeletons indicated that the average estimated stature was 168.32 ± 4.70 for males and 160.45 ± 6.89 cm for females. Our findings indicate that long bone measurements are comparable among remains from each sample. However, sexual dimorphism in estimated stature was clearly greater in recent human remains in comparison to Gilimanuk, Semawang, and Plawangan skeletal remains. Our findings suggest temporal changes in stature in this part of Indonesia.
Key words: sexual dimorphism, stature, Gilimanuk, Plawangan, Semawang
Introduction
Sexual dimorphism characterizes variability in the morphology of different sexes (male and female) in a population. Sexual dimorphism, which exists in human skeletons, can be more prominent in certain parts, and less prominent in other parts, of bones. For example, one study in a Thai sample indicated that the length of the upper limb bone in males was significantly longer in all dimensions compared to females (Duangto and Mahakkanukrauh 2019). Studies on sexual dimorphism of human skeletal remains can also provide information on the variability in biological traits and behaviors, and aid in identifying secular and evolutionary trends of past populations (Kay 1982; Dong 1997). For example, a study of the Pre-Hispanic Maya Coastal Population in Mexico conducted by Wanner et al. (2006), reported sex differences in occupations can be observed from sexual dimorphism in bone structures. Wanner et al. (2006) found a difference in the robustness in the upper limbs caused by the division of daily occupations, such as the use of different tools and methods of carrying heavier burdens by mostly the males. Generally, males look more robust with broader shoulders when compared to females (Wanner et al. 2006). It was also suggested that males tend to be more easily affected by poor nutritional conditions compared to females. When males receive poor nutritional quality, they tend to have a decrease in the length of their long bones, which may affect their stature. Meanwhile, fluctuations in nutritional quality do not necessarily have similar effects in females (Gray and Wolfe 1980).
In archaeology and forensic anthropology, an individual’s stature can be estimated using a regression formula applied to their skeletal remains. Such formulae are typically based on maximum lengths of given long bones, since there is a good correlation between long bone lengths, such as the femur, and living height. Stature estimation regression formulae should be ancestry specific. Estimating stature for remains with Asian ancestry is often done using the Trotter and Glesser (1958) method. There are several other formulae, such as Sangvichien et al. (1985), and Mahakkanukrauh et al. (2011), but these may need further investigation for their application due to some limitations. For example, Sangvichien et al. (1985) used 200 long bones (femur, tibia and fibula) from a Thai and Chinese sample. Only the leg bones, not arm bones (humerus, radius, and ulna), were considered. While the Mahakkanukrauh et al. (2011) study developed a formula for all long bones, it has a high standard error obtained from female skeletal remains. While the regression formula by Trotter and Gleser (1958) is currently considered a better choice in determining individual’s stature in Asia, it must be noted that several caveats have also been noted regarding its suitability in studies on sexual dimorphism (see Jeong and Jantz 2016).
The Gilimanuk (Indonesia) skeletons have received little attention in the bioarcheological literature. Koesbardiati et al. (2013) used Gilimanuk remains to examine past genetic variation in this population. Other studies used these skeletal remains to explain health conditions in the past (Prayudi and Suriyanto 2017; 2018). Indriati (2002) also studied human bones from the Gilimanuk prehistoric population and compared them to the measured stature of students in Yogyakarta and several other populations worldwide. The study found that there was no substantial variation in human stature in Indonesia within two millennia. The stature of Indonesians was intermediate relative to stature of other populations globally; in a similar range with several other Asian groups, including those from Hongkong, Taiwan, Thailand, and India, but lower than Europeans and Americans.
The Semawang site is located in Sanur Beach, Bali, Indonesia. Herbiamami (2014) discovered several ancient people buried at the Semawang site with modified teeth. Aside from that, the Semawang population have a poor life expectancy, with mortality occurring at the age of 40 years or younger. Whereas, the Plawangan site on the northern shore of Java, Indonesia, has received increased attention for Paleometallic artefacts. According to Boedhisampurno (1990), most of the individuals discovered during excavations at the Plawangan site were young adults with an average stature of 160.4 cm. The differences in the skull, bones, and teeth, as well as the altered form of the teeth, bring them closer to the Mongoloid characteristics, yet the Australomelanesoid traits are retained. Damai (2023) concluded that, based on the skeleton, the Plawangan population was an agricultural community, but also relied on the sea for sustenance. Meanwhile, Yuniawan (2002) found that there was no association between health and economic conditions in Plawangan population.
The present study builds on this earlier research to better understand the health and adaptive success of individuals in Indonesia by identifying intrapopulation and evolutionary trends (Khudaverdyan and Hobossyan 2017). While the use of human skeletons from the past as material for medical research has been widely done to determine the history of health and diseases in some countries, in Indonesia this approach is very limited. This study aimed to examine the sexual dimorphism in estimated stature of Gilimanuk, Semawang, and Plawangan and a more recent sample of long bones from Indonesia to discuss whether there has been a temporal trend in health change.
Materials and Methods
Sample
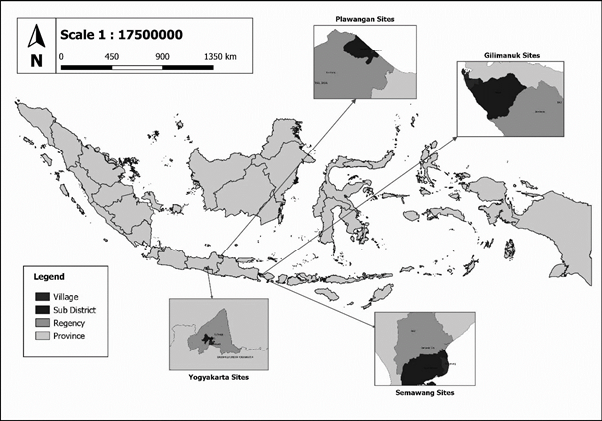
This research was conducted at the Laboratory of Bioanthropology and Paleoanthropology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia where these skeletal remains were stored after exhumation. Data collection was done in 2023. The materials used were human skeletal remains including 44 individual skeletal remains from the Gilimanuk site (16 males, 28 females), nine from the Semawang site (five males, four females), 11 from the Plawangan site (four males, seven females), and nine from recent age (four males, five females). The recent age skeleton samples were anatomical samples stored at the Lab. of Bioanthropology & Paleoanthropology, Faculty of Medicine, Public Health, and Nursing Universitas Gadjah Mada, Indonesia during the Middle of 20th Century. It was ensured that the skeletons had long bones with no or little damage, and that adult age-at-death and sex was estimated, so that stature estimation formulae could be applied. Figure 1 depicts the sites of Gilimanuk, Semawang, and Plawangan on a map of Indonesia.
The Gilimanuk ancient burial site had been used since 750 BCE up to 900 CE based on radiocarbon dating on charcoal and bone fragments of four individuals from the site (Aziz and Faisal 1997). The discovery of the Gilimanuk site was reported by Public Works Office workers while employed at the construction of the Gilimanuk – Singaraja road where they found a large number of pottery shards, with several archaeological objects such as a square pickaxe, animal and human bones in the Cekik Village, Gilimanuk, Bali Province, Indonesia. The first research on this site was conducted in 1962–1963 by Soejono from the Bedulu National Archaeological and Heritage Branch Office in Gianyar. Subsequent studies have continued and uncovered 160 individuals (Soejono 1977).
The other archaeological sites in the present study are the Semawang and Plawangan. Semawang is located about 10 m next to Sanur Beach in Semawang Village, Sanur District, Badung Regency, Bali Province, Indonesia (Harkatiningsih 1990). The Semawang site was first discovered by local people when they dug a septic tank in 1986, followed by a study that resulted in the discovery of the grave. Significant archaeological finds from the site included various kinds of ceramics that vary in age from the 10th to 17th centuries (Harkatiningsih 1990). Whereas, Plawangan is an archaeological site from prehistoric times located in Plawangan and Balongmulyo villages, Kragan, Rembang District, Central Java province, Indonesia covering an area of approximately 90 hectares, and about 500 m from the coastline 4 m above sea level. This site is not only a burial site but also a residential site with relics such as pottery, pendulum nets, hooks and coins. Food remains obtained from the site were shellfish, snails and marine fish which indicated that the people who lived in Plawangan were fishermen (Boedhisampurno 1990; Prasetyo 1995). The Plawangan site was discovered in 1977 by the local community while building the groundwork for the village hall. Subsequently, archaeological survey research was conducted and unearthed many prehistoric graves (Prasetyo 1995).
Procedure
First, we observed the condition of the human skeletons to identify those that are in good condition, then we estimated sex by assessing bones that have sexually dimorphic traits on the pelvis or skull based on Walker in Buikstra and Ubelaker (1994). We used parts of the skull such as the nuchal crest, mastoid process, supraorbital margin, glabella, and mental eminence. Meanwhile, sex estimation using the pelvis was done by examining the greater sciatic notch, ventral arc, subpubic concavity, and ischiopubic ramus.
Second, we estimated the age-at-death by assessing the auricular surface, pubic symphysis, or the condition of the sutures in the skull. Estimation of age-at-death was carried out by examining several criteria, including the degree of closure of the cranial sutures based on Walker in Buikstra and Ubelaker (1994), the degree of fusion of the epiphyses of the bone based on McKern and Stewart (1957), the pattern of changes in the surface of the pubic symphysis based on Todd (1920), and changes in auricular surface based on Lovejoy et al. (1985).
Third, we measured the long bones including: humerus, radius, ulna, femur, tibia, and fibula. To provide validation, measurements were made by two research assistants who had skills and knowledge of measuring human skeletons using calibrated and standardized calipers (GPM, Swiss).
Stature estimation was done by entering the bone length measurements into the regression formula of Trotter and Gleser (1958) as showed in Table 1.
Individual ages were grouped based on a 10-year time span, to see significant growth in stature. These groups were:
1. < 16 years
2. 16 – <20 years
3. 20 – <30 years
4. 30 – <40 years
5. ≥ 40 years
Several bone structures were also observed and measured in addition to the maximum length of the bones (see Table 2). Measurements were done following the procedures of Olivier (1969).
| No. | Bones | Formula |
|---|---|---|
| Single bone | ||
| 1. | Humerus | 2.68 Humerus + 83.19 ± 4.25 |
| 2. | Radius | 3.54 Radius + 82.00 ± 4.60 |
| 3. | Ulna | 3.48 Ulna + 77.45 ± 4.66 |
| 4. | Fibula | 2.40 Fibula + 80.56 ± 3.24 |
| 5. | Tibia | 2.39 Tibia + 81.45 ± 3.27 |
| 6. | Femur | 2.15 Femur + 72.57 ± 3.80 |
| Combined bones | ||
| 7. | Humerus and Ulna | 1.68 (Humerus + Ulna) + 71.18 ± 4.14 |
| 8. | Humerus and Radius | 1.67 (Humerus + Radius) + 74.83 ± 4.16 |
| 9. | Femur and Humerus | 1.22 (Femur + Fibula) + 70.24 ± 3.18 |
| 10. | Femur and Tibia | 1.22 (Femur + Tibia) + 70.37 ± 3.24 |
| Bones | Measurements |
|---|---|
| Humerus | Transverse diameter of diaphysis |
| Maximal length | |
| Radius | Maximal diameters of medial diaphysis |
| Antero-posterior diameters of medial diaphysis | |
| Minimal circumference of diaphysis | |
| Maximal length | |
| Ulna | Minimal circumference of diaphysis |
| Maximal anteroposterior diameters of diaphysis | |
| Maximal transverse diameters of diaphysis | |
| Maximal length | |
| Femur | Trochanter lengths |
| Maximal lengths | |
| Circumference of medial diaphysis | |
| Transverse diameter of medial diaphysis | |
| Transverse sub-trochanteric diameter | |
| Antero-posterior sub-trochanteric diameter | |
| Transverse diameters of head | |
| Sagittal diameters of head | |
| Collo-diaphyseal angle | |
| Divergent angle | |
| Tiba | Maximal length without tibial spine |
| Width of superior epiphysis | |
| Transverse diameter of diaphysis | |
| Maximal length |
Data analyses were performed using descriptive analysis (average, standard deviation (SD), minimum-maximum). Comparative analysis is used to compare the estimated stature based on age and sex of the Gilimanuk, Semawang and Plawangan remains with recent Javanese skeletal remains.
Results
The estimation of stature from the maximum length of long bones of the prehistoric Gilimanuk sample is shown in Table 3, which is separated by sex and categorized into several age-at-death groups. The highest estimated stature is found in male individuals aged 16 – <20 years and in females aged ≥ 40 years. The highest average for males and females was found in the age range of 16 – <20 years (168.74 ± 9.18 cm), with the average estimated stature of males in that age range being the highest average stature in the entire Gilimanuk sample (174.10 ± 9.42 cm). The average estimated stature of males and females is almost similar i.e., approximately 164 cm.
| Age at death | N | Average Estimated Stature | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Male | |||||
| 16 – <20 | 2 | 174.10 | 9.42 | 167.44 | 180.77 |
| 20 – <30 | 10 | 164.40 | 6.77 | 151.23 | 174.75 |
| 30 – <40 | 3 | 160.84 | 5.41 | 157.36 | 167.07 |
| ≥ 40 | 1 | 163.41 | – | 163.41 | 163.41 |
| Total | 16 | 164.88 | 7.23 | 151.23 | 180.77 |
| Female | |||||
| 16 – <20 | 2 | 168.74 | 9.18 | 162.25 | 175.24 |
| 20 – <30 | 12 | 163.12 | 5.99 | 152.10 | 171.21 |
| 30 – <40 | 8 | 165.11 | 3.45 | 159.17 | 170.56 |
| ≥ 40 | 6 | 162.78 | 8.11 | 155.28 | 178.37 |
| Total | 28 | 164.01 | 5.98 | 152.09 | 178.37 |
Age at death in year; N: number of specimens; SD: standard deviation; estimated stature in cm
Table 4 shows estimated stature of males and females from Semawang and Plawangan skeletal remains. It can be seen that in contrast to Gilimanuk, the highest estimated stature for males of Semawang and Plawangan remains is in the age range of ≥40 which also has the highest average estimated stature (166.31 ± 1.52 cm). Meanwhile, females, the highest average estimated stature was found in the age range of 20 – <30 years (160.51 cm). Males are about 3 cm taller than females (Table 4).
| Age at death | N | Average Estimated Stature | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Male | |||||
| 20 – <30 | 2 | 160.40 | 3.80 | 157.71 | 163.09 |
| 30 – <40 | 1 | 159.57 | – | 159.57 | 159.57 |
| ≥40 | 2 | 166.31 | 1.52 | 165.24 | 167.39 |
| Total | 5 | 162.60 | 3.97 | 157.71 | 167.39 |
| Female | |||||
| 16 – <20 | 2 | 159.58 | 2.64 | 157.40 | 158.88 |
| 20 – <30 | 1 | 160.51 | – | 160.51 | 160.51 |
| 30 – <40 | 3 | 158.27 | 0.77 | 157.71 | 161.45 |
| Total | 6 | 159.08 | 1.59 | 157.40 | 161.45 |
Age at death in year; N: number of specimens SD: standard deviation; estimated stature in cm
In our sample of recent population, the age-at-death range cannot be determined because there was no available data from the collections and only a little information about age can be identified. Males are on average 8 cm taller than females (Table 5).
| N | Average Stature | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Males | 5 | 168.32 | 4.70 | 161.65 | 174.89 |
| Females | 4 | 160.45 | 6.89 | 150.76 | 166.73 |
N: number of specimens; SD: standard deviation; estimated stature in cm
Tables 6–7 present the sexual dimorphism characteristics of upper and lower limb bone remains from Gilimanuk, Semawang, Plawangan, and recent humans. Several bone structures in humerus, radius, ulna, femur, and tibia were compared among males and females from Gilimanuk, Semawang, Plawangan, and recent human remains. The measures vary considerably among population remains; however, the values are comparable. Humerus, radius, ulna, and tibia of males had greater maximum length of long bones than females in all population remains. The differences were not seen in femur measurements which had almost comparable length between males and females in all sample populations. More detailed structures in each of the long bones also were highly varied between males and females in the population remains. For examples, transversal diameter of diaphysis of humerus were greater in females than males in all populations except in the Semawang remains. The minimal circumference of diaphysis of radius, however, was greater in males than females in all populations, except in the Plawangan remains.
| Bone and structures | Gilimanuk | Semawang | Plawangan | Recent | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | |
| Humerus | ||||||||
| Transverse diameter of diaphysis | 21.7 (3.8) | 22.9 (14.1) | 18.9 (3.1) | 16.0 (1.9) | 17.2 (3.9) | 19.0 (1.5) | 16.7 (2.9) | 18.4 (2.3) |
| Maximal length | 308.0 (16.1) | 294.3 (19.9) | 290.0 | 168.0 | 280.5 | – | 319.2 (11.3) | 280.8 (17.7) |
| Radius | ||||||||
| Maximal diameter of medial diaphysis | 15.9 (3.5) | 15.8 (6.3) | 16.2 (2.4) | 12.0 | 14.0 (1.4) | 16.4 (1.7) | 13.1 (0.7) | 9.5 |
| Antero-posterior diameter of medial diaphysis | 15.6 (7.4) | 12.1 (2.2) | 11.4 (1.3) | 9.5 | 10.0 (1.0) | 10.8 (0.9) | 10.3 (0.8) | 7.5 |
| Minimal circumference of diaphysis | 47.0 (11.6) | 42.8 (6.4) | 46.3 (1.9) | 40 | 39.8 (4.5) | 45.0 (3.5) | 41.6 (3.1) | 36.0 |
| Maximal length | 251.8 (23.3) | 214.1 (56.6) | 235.0 | – | 222.5 | 240.0 | 245.4 (10.5) | 196.0 |
| Ulna | ||||||||
| Minimal circumference of diaphysis | 40.2 (8.2) | 39.1 (6.6) | 37.3 (1.1) | 33.0 | 26.3 (12.9) | 37.0 (1.7) | 35.5 (1.5) | 29.6 (3.6) |
| Maximal antero-posterior diameter of diaphysis | 24.7 (8.8) | 31.2 (9.9) | 32.0 | 1.00 | 31.4 (3.7) | 29.6 (2.6) | 33.5 (2.1) | 29.8 (1.1) |
| Maximal transverse diameter of diaphysis | 21.1 (6.8) | 26.4 (9.3) | 22.0 | 15.0 | 26.5 (0.6) | 20.4 (3.6) | 24.1 (1.0) | 21.8 (0.8) |
| Maximal length | 239.8 (18.8) | 254.3 (23.0) | 250.0 | – | 240.5 | 235.7 (8.3) | 264.6 (10.4) | 241.8 (7.4) |
Avg: average; SD: standard deviation; measurement unit is in mm
| Bone and structures | Gilimanuk | Semawang | Plawangan | Recent | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | Avg (SD) | |
| Femur | ||||||||
| Trochanter length | 403.0 | 406.3 (18.8) | 353.0 (7.3) | – | 336.5 | 33.7.4 | 345.3 (27.2) | 341.3 (34.9) |
| Circumference of medial diaphysis | 90.2 (6.5) | 83.3 (7.3) | 85.8 (6.6) | 77.0 (5.2) | 76.0 (1.7) | 84.3 (5.0) | 74.6 (7.0) | 76.0 (9.2) |
| Transverse diameter of medial diaphysis | 25.8 (2.1) | 27.9 (13.9) | 25.9 (3.1) | 23.7 (1.8) | 24.5 (1.5) | 24.9 (2.0) | 22.3 (2.8) | 23.8 (1.6) |
| Transverse sub-trochanteric diameter | 33.1 (3.1) | 34.9 (17.1) | 32.2 (1.6) | 27.0 | 26.3 (1.5) | 28.9 (3.6) | 25.4 (0.5) | 26.8 (2.7) |
| Antero-posterior sub-trochanteric diameter | 30.3 (4.9) | 28.5 (4.0) | 28.4 (4.7) | 22.0 | 22.0 (1.9) | 2.53 (2.4) | 22.6 (1.6) | 20.7 (4.0) |
| Transverse diameter of head | 43.8 (3.8) | 42.5 (3.0) | 45.5 (2.1) | – | 39.5 | 41.8 (2.3) | 40.5 (5.4) | 3.93 (0.49) |
| Sagittal diameter of head | 44.0 (3.6) | 42.6 (2.9) | 44.5 | – | 39.5 | 41.3 (2.2) | 40.5 (4.6) | 3.88 (0.50) |
| Collo–diaphyseal angle | 134.00º (8.31 º) | 128.15º (6.79 º) | – | – | – | 140º | 135º | 129º |
| Divergent angle | 7.25 (1.50 º) | 11.50º (2.65 º) | – | – | – | 10º | 12º | 9º |
| Maximal length | 420 | 430 | 382.7 (74.0) | 328.3 (55.3) | 400.0 | 416.3 (14.4) | 407.1 (32.1) | 400.2 (43.2) |
| Tibia | ||||||||
| Maximal length without tibial spine | 338.3 (14.7) | 344.3 (18.0) | – | – | – | 336.0 | 365.0 (16.6) | 323.3 (38.2) |
| Width of superior epiphysis | 58.0 (18.3) | 71.8 (7.6) | 62.5 | – | 61.0 | 61.2 (6.8) | 71.2 (1.6) | 62.0 (6.1) |
| Transverse diameter of diaphysis | 31.0 (4.7) | 34.6 (8.5) | 29.5 | – | 24.5 | 27.0 | 25.8 (1.6) | 25.7 (3.8) |
| Maximal length | 353.5 | 348.2 (18.2) | 370 | – | – | 346.0 | 377.3 (20.4) | 334.0 (33.5) |
Avg: average; SD: standard deviation; measurement unit is in mm
Discussion
Sexual dimorphism refers to the systemic biological differences between males and females, which can be measured and seen in estimated stature and bone structures. One of the common types of archeological evidence to find is that males are almost always taller than females. This is often ascribed to what is known about living males, such as that they tend to have a stocky face, muscular body, and are generally physically stronger and faster than females (Frayer and Wolpoff 1985). In our study on human skeletal remains from Indonesia, sexual dimorphism in estimated stature of Gilimanuk, Semawang, and Plawangan was obscure compared with the recent sample, where sexual dimorphism was more obvious. Nonetheless, long bones showed more obvious sexual dimorphism in all population groups.
It is believed that stature is strongly influenced by genetics. However, one possible confounding cause has emerged that could affect stature, i.e., nutrition (Eveleth 1975; Gray and Wolfe 1980). Overall, genetics and nutrition play the most important role in human growth, but when considered in other cultural and regional contexts, it can be seen that there are several other factors such as climate, marriage, or even access to the basic requirements of nutrients.
The Gilimanuk, Semawang, and Plawangan populations have genetic and physical traits as Mongoloid. They lived at around the same period and belonged to the Paleometallic Age and Austronesian civilizations. They dwell by the coast (Soejono 1977; Boedhisampurno 1990; Harkatiningsih, 1990, Aziz and Faisal 1997; Koesbardiati et al. 2013; Prayudi and Suriyanto, 2017, 2018). It is possible that in the past in Gilimanuk there were conditions when the community was malnourished so that the stature of males at that time did not reach the maximum when compared to the stature in the recent period. In this study, it was seen that there was a change in the estimated stature of the past sample when compared to modern, while modern males get taller (by 5.44 cm), modern females get shorter (by 3.65 cm). In the Gilimanuk community, males have an average estimated stature of 164.88 cm, while they were 162.60 cm in Semawang. This trend may indicate that in the past in Gilimanuk and Semawang, there may have been conditions that made it difficult for them to access nutrition. Sexual dimorphism among ancient populations has been reported in ancient European populations by Frayer (1980). It was noted that European Upper Paleolithic groups exhibited stronger sexual dimorphism than their Mesolithic and Neolithic descendants, particularly in the cranial skeleton. This decrease in sexual dimorphism towards more recent populations was linked to changes in technical processes connected with hunting of prey animals. The same process was proposed to have occurred between Mesolithic and Neolithic European populations, as well as between Neolithic and current European populations, indicating a stronger correlation with changes in women (Frayer 1980).
Sexual dimorphism is diminished in hominin descendants, and is more visible or higher in more ancient populations, in terms of skeletal, cranial, and dental dimensions (Suriyanto 2006; 2009). The degree of sexual dimorphism in hominin body size has shifted over the last three million years, from 100% in baboons and gorillas to 20% – 40% in current human populations (Frayer and Wolpoff 1985). Our study found that sexual dimorphism in the Gilimanuk sample was not obvious. This was seen in the average estimated stature difference between males and females, which was only 0.87 cm when compared with 7.87 cm different in modern males and females. When we compare the average stature of males and females across Gilimanuk, Semawang, and Plawangan, there was not much of a difference either (Tables 3 and 5). Changes in nutrition can cause wider sexual dimorphism (Chen et al. 2022; Pontifex et al. 2024) as shown in our more recent population. Males have a tendency to get more nutrition, because, for example, females tend to get diseases related to lack of nutrition, especially when there is a disaster (Rivers 1982). The absence of sexual dimorphism in bones from the past may also occur because there are similarities in activities between males and females (Murdock and Provost 1973). This seen in the robustness of the lower body of the Modern Hunter Gatherer from Australia which indicates both males and females have a high level of mobility (Carlson et al. 2007; Herrerín and Carmenate 2022). However, there are differences in the upper body, which based on ethnographic data may be due to differences in occupation (Carlson et al. 2007). There might be various explanations for the lack of prominent sexual dimorphism in prehistoric populations, such as equality of work between males and females and equal access to nourishment. Whereas, several causes of sexual dimorphism in the modern society may include unequal work between males and females as well as a lack of access to nutrients (Frayer and Wolpoff 1985; Kirchengast 2014).
Overall, our observations on the long bone structures showed the existence of sexual dimorphism among the skeletal remains in Indonesia. The humerus transverse diameter of the female population of Gilimanuk, Plawangan, and recent skeletons are greater than the male humerus, while the humerus transverse diameter of the Semawang male population are greater than the female humerus. There is an increase in the maximum length of the humerus of Gilimanuk, Semawang, Plawangan, and recent Indonesian males. The osteometric measurements of the radius and ulna of males appeared to be greater for all populations, but the differences in the osteometric measurements of the radius and ulna of males and females in the populations were relatively inconspicuous. The maximal length of radius between male and female recent populations is relatively greater compared to the ancient populations of Indonesia. Whereas, lengths, diameters, and angle of the female femur were relatively greater in ancient populations, the lengths, diameters, and angle of the male femur were greater in the recent population. Femur circumference is relatively greater in males Gilimanuk and Semawang populations than of Semawang and recent populations. Herrerín and Carmenate (2022) found that the robustness in legs showed higher dimorphic trends than in the arms in Santa Clara Necropolis skeletal remains. It was thought that there might be a similar manipulation activity between males and females in that population.
The tibia remains of Semawang and Plawangan populations are difficult to measure all osteometric variables because most of them are fragmentary since at the archeological sites, hence, the measurements were done only on the ancient population of Gilimanuk. In general, recent Indonesian tibial osteometric measurements show relatively less pronounced sexual dimorphism than the ancient Gilimanuk population. It may be that daily activities related to the sexual division of labor in tibial function were more pronounced in ancient populations than in more modern populations (Ruff 1987).
Concerning the sexual dimorphism in long bone structures, sexual division of labor can also be seen from the measurements of the actual size of the bones. For example, sexual dimorphism was found in the Pre-Hispanic Maya Coastal Population of Mexico where there was a difference in robustness in the upper limbs caused by the division of labor such as the use of different tools and carrying a heavier burden by males. In addition, the lower limbs also showed differences in work groupings, so there are differences in the structures of the bones. Additionally, in morphology, males look more robust with broader shoulders when compared to females. This pronounced difference is probably caused by males frequently traveling and carrying heavy loads (Wanner et al. 2006).
The strength of this study was the antiquity of the materials of Gilimanuk human remains and the methods of sex and age estimation. Sex identification used the Walker method in Buikstra and Ubelaker (1994). This method uses skull and pelvis, both of which are the parts that show the most sex differences. Individual age at death was identified using various methods such as Todd (1920), McKern and Stewart (1957), Lovejoy et al. (1985), and Walker in Buikstra and Ubeler (1994). Age identification is done using various methods because not all bones in an individual can be found. More recent methods assessed the use of osteometric measurement and indices; however, the results were less reliable than morphological characteristic observation (Barroso Flamino et al. 2020). The use of these two parts of the skeletons is also to reduce identification errors. Nonetheless, several limitations were found in this study, including the limited individual number of specimens and the remains were not comparably distributed in the observed age ranges. The limited number of specimens may hinder the representation of the whole population. Future study should investigate additional characteristics such as socioeconomic, culture, activities, and environment of the specimens.
Conclusions
In conclusion, long bone structure measurements are comparable among remains from each sample. Long bone structures, however, revealed more obvious sexual dimorphism in all skeletal remains. This finding may be due to the difference in household tasks between males and females. Additionally, there is a trend that males tend to get more nutrition and get it earlier in their development than females. Sexual dimorphism in estimated stature was clearly greater in recent human remains in comparison to Gilimanuk, Semawang, and Plawangan skeletal remains. This showed that the possibility of accessing nutrients by each individual tends to be similar. In addition, the work done during that time tended to be alike between males and females, indicating an equal division of labor in both sexes. Nonetheless, the average estimated stature and age at death varied among the premodern remains.
References
Aziz FA, Faisal W. 1997. Pertanggalan radiocarbon rangka manusia situs Gilimanuk, Bali. Naditira Widya 2:52–62.
Barroso Flamino C, Oliveira D, Ferreira L, Martins M, Santos R., Laureano R, Nunes T, Bento B, Santos R, Palmela Pereira C. 2020. Osteometric and osteomorphological sex estimation from the os coxa in an archaeological population related to the 1755 Earthquake of Lisbon. Bull Int Assoc Paleodont 14(1):32–39.
Boedhisampurno S.1990. Temuan sisa manusia dari situs kubur Paleometalik Plawangan, Rembang, Jawa Tengah, In RP Soejono, NA Subagus, Nurhadi, HM Ambary, S Satari, DD Bintarti, and ES Hardiati, editors. Proceedings Analisis Hasil Penelitian Arkeologi I. Jakarta: Pusat Penelitian Arkeologi Nasional, Departemen Pendidikan dan Kebudayaan. 125–164.
Buikstra JE, Ubelaker DH. 1994. Standards for data collection from human skeletal remains. Arkansas Archaeological Survey, Arkansas.
Carlson KJ, Grine FE, Pearson OM. 2007. Robusticity and sexual dimorphism in the postcranium of modern hunter-gatherers from Australia. Am J Phys Anthropol 134:9–23. https://doi.org/10.1002/ajpa.20617
Chen Y, Kim M, Paye S et al. 2022. Sex as a biological variable in nutrition research: from human studies to animal models. Annu Rev Nutr 42: 227–250. https://doi.org/10.1146/annurev-nutr-062220-105852
Damai AH. 2023. Deskripsi paleopatologi gigi manusia purba berdasarkan aktivitas di Situs Plawangan. Unpublished bachelor dissertation. Program Studi Arkeologi Universitas Udayana. Dong Z. 1997. Mixture analysis and its preliminary application in archaeology. J Archaeol Sci I:141–161. https://doi.org/10.1006/jasc.1996.0100
Duangto P, Mahakkanukrauh P. 2020. Sex estimation from upper limb bones in sex dimorphism in a Thai population. Anat Cell Biol. 53(1):36–43. https://doi.org/10.5115/abc.19.179
Eveleth PB. 1975. Differences between ethnic groups in sex dimorphism of adult height. Ann Hum Biol 2(1):35–9. https://doi.org/10.1080/03014467500000541
Frayer D.W. 1980. Sexual dimorphism and cultural development in the Late Pleistocene and Holocene of Europe. J Hum Develop 9(5): 399–415.
Frayer DW, Wolpoff MH. 1985. Sexual dimorphism. Annu Rev Anthropol 14:429–473. https://doi.org/10.1146/annurev.an.14.100185.002241
Gray JP, Wolfe LD. 1980. Height and sexual dimorphism of stature among human societies. Am J Phys Anthropol 53(3):441–56. https://doi.org/10.1002/ajpa.1330530314
Harkatiningsih MN. 1990. Jenis dan Peletakan Bekal Kubur di Situs Semawang dan Selayar: Pola Kubur dari Abad ke-14–19. Analisis Hasil Penelitian Arkeologi I. Departemen Pendidikan dan Kebudayan, Jakarta.
Herbiamami ML. 2014. Status Kesehatan manusia di Situs Semawang, Bali (Kajian paleopatologi rangka dan gigi). Unpublished bachelor dissertation. Program Studi Arkeologi Universitas Gadjah Mada.
Herrerín J, Carmenate M. 2022. Selular dimorphism of a Mudejar Necropolis of Santa Clara (Cuéllar, Segovia, S. XIV and S. XV). Anthropologie (Brno) 60(1):0–0. https://doi.org/10.26720/anthro.21.06.10.1
Indriati E. 2002. Stature in Yogyakarta’s students and prehistoric Balinese circa 110 A.C. Berkala Ilmu Kedokteran 34(1):1–7.
Ipiña SL, Durand AI. 2010. Some considerations on the use of the quotient of sample means as a measure of sexual dimorphism. Anthropologie (Brno) 48(1):1–4.
Jeong Y, Jantz LM. 2016. Caveats in using Trotter and Gleser’s (1958) Asian equations for stature estimation. Korean J Phys Anthropol 29(3): 81–91.
Kay RF. 1982. Sivapithecus simonsi a new species of Miocene hominoid, with comments on the phylogenetic status of Ramapithecinae. Int J Primatol 3:113–173. https://doi.org/10.1007/BF02693493
Khudaverdyan A, Hobossyan SG. 2017. Bioarchaeological evidence for the health status of a late Bronze Age and Early Iron Age Bakheri Chala population (Armenia). Anthropologie (Brno) 55(3): 319–336.
Kirchengast S. 2014. Human sexual dimorphism—a sex and gender perspective. Anthropol Anz 71(1–2):123–33. https://doi.org/10.1127/0003-5548/2014/0376
Koesbardiati T, Yudianto A, Murti DB, Suriyanto RA. 2013. Loci STR codis (THO1,TPOX) genetic variation on Gilimanuk Man (Bali Islands). Berkala Arkeologi 33(2). https://doi.org/10.30883/jba.v33i2.11
Lovejoy CO, Meindl RS, Pryzbeck TR, Mensforth RP. 1985. Chronological metamorphosis of the auricular surface of the ilium: A new method for the determination of adult skeletal age at death. Am J Phys Anthropol 1(68):15–28. DOI: https://doi.org/10.1002/ajpa.1330680103
Mahakkanukrauh P, Khanpetch P, Prasitwattanseree S, Vichairat K, Case DT. 2011. Stature estimation from long bone lengths in a Thai population. Forensic Sci Int 210(279):e1–7. https://doi.org/10.1016/j.forsciint.2011.04.025
Mckern TW, Stewart TD. 1957. Skeletal age changes in young American males. Natick, MA: Quartermaster Research and Development Command Technical Report EP-45.
Murdock GP, Provost C. 1973. Factors in the division of labor by sex: A cross-cultural analysis. Ethnology 12(2):203–225.
Pontifex MG, Vauzour D, Muller M. 2024. Sexual dimorphism in the context of nutrition and health. Proc Nutr Soc. 83(2):109–119. https://doi.org/10.1017/S0029665123003610
Prasetyo B. 1995. Laporan Penelitian Situs Plawangan, Rembang, Jawa Tengah (1980–1993). Proyek Penelitian Purbakala Nasional. Pusat Penelitian Arkeologi Nasional. Departemen Pendidikan dan Kebudayaan, Jakarta.
Prayudi A, Suriyanto RA. 2017. Osteobiografi individu nomor 38 dari situs Prasejarah Gilimanuk. Amerta 35(1):19–32. https://doi.org/10.24832/amt.v35i1.139
Prayudi A, Suriyanto RA. 2018. GLM LVI: Tinjauan Osteoarkeologis atas sebuah rangka dari Gilimanuk. Forum Arkeologi 31(2):105–116. https://doi.org/10.24832/fa.v31i2.526
Rivers J. 1982. Women and children last: An essay on sex discrimination in disasters. Disasters 6: 256–267. https://doi.org/10.1111/j.1467-7717.1982.tb00548.x
Ruff C. 1987. Sexual dimorphism in human lower limb bone structure: Relationship to subsistence strategy and sexual division of labor. J Hum Evol 16(5):391–416. https://doi.org/10.1016/0047-2484(87)90069-8
Sangvichien SJ, Srisurin V, Wattanakyinsakul V. 1985. Estimation of stature of Thai and Chinese from the length of femur, tibia and fibula. Siriraj Hosp Gaz 37:215–8.
Soejono RP. 1977. Sistim-sistim penguburan pada akhir masa prasejarah di Bali. Unpublished dissertation (PhD), Program Studi Arkeologi Universitas Indonesia.
Suriyanto RA. 2005. Kajian perbandingan karakteristik epigenetis populasi tengkorak manusia paleometalik Gilimanuk (Bali) dan Liang Bua, Lewoleba, Melolo dan Ntodo Lesah (Nusa Tenggara Timur). Unpublished thesis (Master), Program Pascasarjana Antropologi Universitas Gadjah Mada.
Suriyanto R.A. 2006 Dimorfisme seksual dalam karakteristik epigenetis upper viscerocranium dari sampel tengkorak manusia Gilimanuk (Bali). Berkala Arkeologi 26 (2): 85–113.
Suriyanto R.A. 2009 Human sexual dimorphism: From an evolutionary perspective to practical overview. Berkala Ilmu Kedokteran 41 (4): 194–201.
Todd TW. 1920. Age changes in the pubic bone. I. The male white pubis. Am J Phys Anthropol 1(3):285–334. https://doi.org/10.1002/ajpa.1330030301
Trotter M, Gleser GC. 1958. A reevaluation of estimation of stature based on measurements of stature taken during life and of long bones after death. Am J Phys Anthropol 1(16):79–123. https://doi.org/10.1002/ajpa.1330160160
Wanner IS, Sosa TS, Alt KW, Blos VT. 2006. Lifestyle, occupation, and whole bone morphology of the Pre-Hispanic Maya coastal population from Xcambo, Yucatan, Mexico. Int J Osteoarchaeol. https://doi.org/10.1002/oa.873
Yuniawan AA. 2022. Paleopatologi manusia Plawangan ekskavasi tahun 1978: kaitan antara bekal kubur dan penyakit. Unpublished bachelor dissertation. Program Studi Arkeologi Universitas Gadjah Mada.
Final information
Ethics statement
Ethics approval for this study was obtained from the Medical and Health Research Ethics Committee Faculty of Medicine, Public Health, and Nursing Universitas Gadjah Mada, Indonesia (KE/FK/1913/EC/2023).
Publication statement
We confirm that the paper has not been previously published or concurrently submitted to an editorial office of another journal, and also that it is approved by all authors for publication.
Acknowledgements
This work received funding from the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada. We are grateful to the research assistants, Fidelis and Novi, for their work. We also thank the laboratory technicians for assisting with the data collection.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Authors’ contribution
JH: research concept and design, data analysis and interpretation, article writing, critical revision of the article for important intellectual content, provision of study materials, statistical expertise, data collection and compilation, final approval of the article; AP: data collection and compilation, technical and logistic support, article writing, data analysis; NTR: critical revision of the article for important intellectual content, provision of study materials, data collection and compilation, approval of the article. NHF: data collection and compilation, administrative, data analysis; RAS: critical revision of the article for important intellectual content, data collection and compilation, technical, or logistic support.
Corresponding author
Lab. of Bioanthropology & Paleoanthropology, Department of Nutrition and Health, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Farmako St, Sekip, Yogyakarta 55281, Indonesia, e-mail: janatin.hastuti@ugm.ac.id